
Covalent Bonds
4 min read•december 1, 2021
Saarah Hasan
Saarah Hasan
Types of Covalent Bonds and How They Form
Chemical Bonding
Chemical bonding is essentially the attractive force that holds atoms together in a compound. The main types of bonding are: ionic, covalent, and metallic. In this article, we're going to be taking a closer look at covalent bonds.
Quick note: many representative elements follow the octet rule, which means they need to attain at least a share of 8️⃣ electrons in their valence shells when they form compounds. Another thing to remember is that there has to be a certain energy advantage for compounds to form!
Sharing of Electrons
Covalent bonding occurs when two atoms share one or more electron pairs.
1️⃣electron pair: single covalent bond
2️⃣electron pairs: double covalent bond
3️⃣electron pairs: triple covalent bond
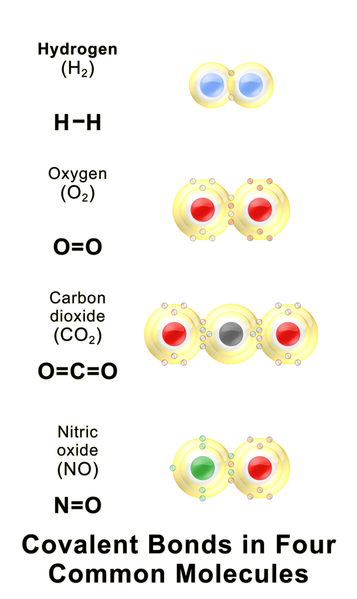
Image from Wikimedia Commons
The bonded arrangement of the compound has lower energy and, as a result, is more stable than the situation where the atoms of the compounds would be separated.
In covalent bonding, atoms tend to achieve noble gas configuration - their valence shells tend to get filled up. Elements are more likely to form a covalent bond when the electronegativity difference between 2 elements is small, and when two nonmetals bond.
Formation of a Covalent Bond
When two atoms start getting close, the electrons on each atom begin to feel the other nucleus pull. This causes the electron density to shift to the region between the two nuclei. The energy of the system starts to decrease until it reaches a minimum. The distance where the energy hits a minimum corresponds to the most stable arrangement and represents the bond length. If we try to decrease the distance further, the system will become less stable due to repulsive forces between the two nuclei.
Polarity
When two different atoms share electrons, the difference in electronegativity isn't 0, and a polar covalent bond forms. In simpler terms, an unequal sharing of the electron pairs results in a polar covalent bond. Equal electron sharing results in a nonpolar covalent bond.
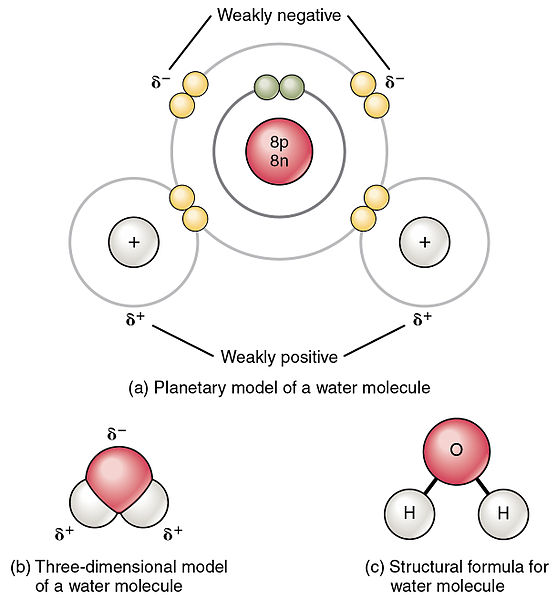
Image from Wikimedia Commons
When determining whether a molecule is polar, we have to keep two things in mind: there's at least one polar bond or one lone pair on the central atom, and the arrangements of the polar bonds will determine if the molecule is polar or not. Do they cancel each other out or not?
We usually look at this with the help of Lewis structures.
Covalent Bonding and Intermolecular Forces
Intermolecular forces (IMF) are the forces acting among the particles of a compound. Physical properties such as boiling point, melting point, and vapor pressure depend on these forces. We're going to explore a little bit of intermolecular forces-hydrogen bonding, dipole-dipole interactions, dispersion forces- that occur in covalent molecules.
Hydrogen Bonding
Strong interactions among polar covalent molecules containing Hydrogen directly attached to 1 of the three small electronegative elements: Oxygen, Nitrogen, Fluorine. (Hydrogen atoms just want to have FON!)
Essentially a powerful dipole-dipole force
Drastically increases the boiling point
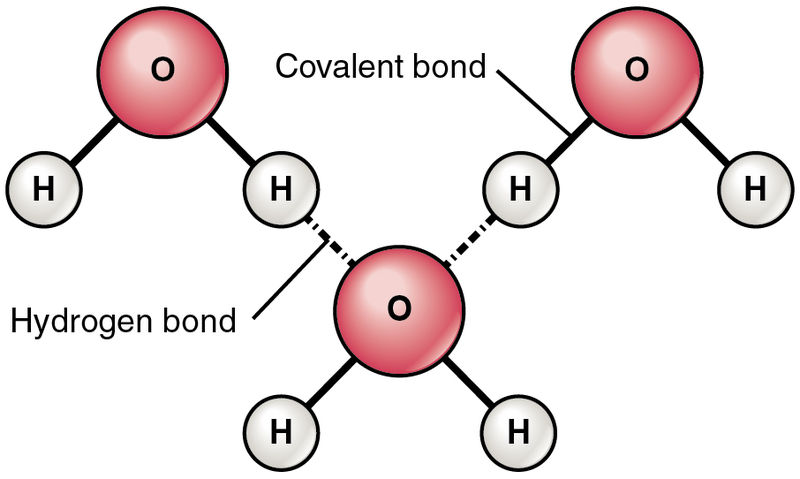
Image from Wikimedia Commons
Dipole-Dipole Interactions
Strong interactions that occur between polar covalent molecules
Occurs due to the attraction of the positive end of a polar molecule to the negative end of another
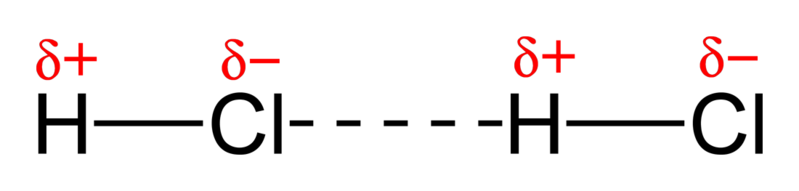
Image from Wikimedia Commons
Dispersion Forces
Very weak attractive forces between short-lived, temporary dipoles
Present for all types of molecules, it's the ONLY intermolecular force for nonpolar covalent molecules.
It occurs from the attraction of the positively charged nucleus of 1 atom to the electron cloud of another, inducing temporary dipoles in surrounding atoms.
Generally stronger for large molecules.
Practice Problems
What intermolecular forces does CO2 have?
CO2 is nonpolar covalent: it has London dispersion forces
What intermolecular forces does NH3 have?
NH3 is polar covalent because of the lone pair on nitrogen; it has dipole-dipole forces and hydrogen bonding (remember hydrogens just want to have "FON"!)
What intermolecular forces does NaCl have?
NaCl is not a covalent molecule! It is an ionic compound, so it has ion-ion interactions.
Liquid State
The variation in properties of liquids, such as boiling point, surface tension, vapor pressure, etc., depends on the nature of the intermolecular forces among the liquid points.
Vapor pressure is the partial pressure exerted on the surface of a substance by its vapor that is in dynamic equilibrium (when the rate of evaporation of water equals rate of its condensation) with the condensed phase in a closed container.
The key thing to remember: as the strengths of our intermolecular forces decrease, vapor pressure increases.
Covalent Bonds
4 min read•december 1, 2021
Saarah Hasan
Saarah Hasan
Types of Covalent Bonds and How They Form
Chemical Bonding
Chemical bonding is essentially the attractive force that holds atoms together in a compound. The main types of bonding are: ionic, covalent, and metallic. In this article, we're going to be taking a closer look at covalent bonds.
Quick note: many representative elements follow the octet rule, which means they need to attain at least a share of 8️⃣ electrons in their valence shells when they form compounds. Another thing to remember is that there has to be a certain energy advantage for compounds to form!
Sharing of Electrons
Covalent bonding occurs when two atoms share one or more electron pairs.
1️⃣electron pair: single covalent bond
2️⃣electron pairs: double covalent bond
3️⃣electron pairs: triple covalent bond

Image from Wikimedia Commons
The bonded arrangement of the compound has lower energy and, as a result, is more stable than the situation where the atoms of the compounds would be separated.
In covalent bonding, atoms tend to achieve noble gas configuration - their valence shells tend to get filled up. Elements are more likely to form a covalent bond when the electronegativity difference between 2 elements is small, and when two nonmetals bond.
Formation of a Covalent Bond
When two atoms start getting close, the electrons on each atom begin to feel the other nucleus pull. This causes the electron density to shift to the region between the two nuclei. The energy of the system starts to decrease until it reaches a minimum. The distance where the energy hits a minimum corresponds to the most stable arrangement and represents the bond length. If we try to decrease the distance further, the system will become less stable due to repulsive forces between the two nuclei.
Polarity
When two different atoms share electrons, the difference in electronegativity isn't 0, and a polar covalent bond forms. In simpler terms, an unequal sharing of the electron pairs results in a polar covalent bond. Equal electron sharing results in a nonpolar covalent bond.

Image from Wikimedia Commons
When determining whether a molecule is polar, we have to keep two things in mind: there's at least one polar bond or one lone pair on the central atom, and the arrangements of the polar bonds will determine if the molecule is polar or not. Do they cancel each other out or not?
We usually look at this with the help of Lewis structures.
Covalent Bonding and Intermolecular Forces
Intermolecular forces (IMF) are the forces acting among the particles of a compound. Physical properties such as boiling point, melting point, and vapor pressure depend on these forces. We're going to explore a little bit of intermolecular forces-hydrogen bonding, dipole-dipole interactions, dispersion forces- that occur in covalent molecules.
Hydrogen Bonding
Strong interactions among polar covalent molecules containing Hydrogen directly attached to 1 of the three small electronegative elements: Oxygen, Nitrogen, Fluorine. (Hydrogen atoms just want to have FON!)
Essentially a powerful dipole-dipole force
Drastically increases the boiling point

Image from Wikimedia Commons
Dipole-Dipole Interactions
Strong interactions that occur between polar covalent molecules
Occurs due to the attraction of the positive end of a polar molecule to the negative end of another

Image from Wikimedia Commons
Dispersion Forces
Very weak attractive forces between short-lived, temporary dipoles
Present for all types of molecules, it's the ONLY intermolecular force for nonpolar covalent molecules.
It occurs from the attraction of the positively charged nucleus of 1 atom to the electron cloud of another, inducing temporary dipoles in surrounding atoms.
Generally stronger for large molecules.
Practice Problems
What intermolecular forces does CO2 have?
CO2 is nonpolar covalent: it has London dispersion forces
What intermolecular forces does NH3 have?
NH3 is polar covalent because of the lone pair on nitrogen; it has dipole-dipole forces and hydrogen bonding (remember hydrogens just want to have "FON"!)
What intermolecular forces does NaCl have?
NaCl is not a covalent molecule! It is an ionic compound, so it has ion-ion interactions.
Liquid State
The variation in properties of liquids, such as boiling point, surface tension, vapor pressure, etc., depends on the nature of the intermolecular forces among the liquid points.
Vapor pressure is the partial pressure exerted on the surface of a substance by its vapor that is in dynamic equilibrium (when the rate of evaporation of water equals rate of its condensation) with the condensed phase in a closed container.
The key thing to remember: as the strengths of our intermolecular forces decrease, vapor pressure increases.

Resources
© 2024 Fiveable Inc. All rights reserved.
AP® and SAT® are trademarks registered by the College Board, which is not affiliated with, and does not endorse this website.