Calculating entropy changes is crucial for understanding thermodynamic processes. This topic explores how to quantify entropy changes in various scenarios, from reversible to irreversible processes, using mathematical formulas and concepts like the Clausius inequality.
The section covers entropy changes under specific conditions, such as isothermal, isobaric, isochoric, and adiabatic processes. It also introduces temperature-entropy diagrams as a visual tool for analyzing thermodynamic systems and their entropy changes.
Entropy Changes in Different Processes
Calculating Entropy Change
- Entropy change quantifies the change in disorder or randomness of a system during a process
- Calculated using the integral , where is the reversible heat transfer and is the absolute temperature
- Entropy is a state function, meaning the change in entropy depends only on the initial and final states, not the path taken between them
- SI unit for entropy is joules per kelvin (J/K)
Reversible and Irreversible Processes
- Reversible processes occur infinitely slowly, allowing the system to remain in equilibrium with its surroundings at all times
- Examples include isothermal expansion of an ideal gas, melting of ice at its melting point
- Irreversible processes occur rapidly, causing the system to deviate from equilibrium with its surroundings
- Examples include spontaneous heat transfer from hot to cold objects, expansion of a gas into a vacuum
- Entropy change for a reversible process is given by , while for an irreversible process, it is

Clausius Inequality
- The Clausius inequality states that for any cyclic process, the integral of is always less than or equal to zero:
- For a reversible cyclic process, the equality holds:
- The Clausius inequality demonstrates that the entropy of an isolated system never decreases, consistent with the second law of thermodynamics
Entropy Changes Under Specific Conditions
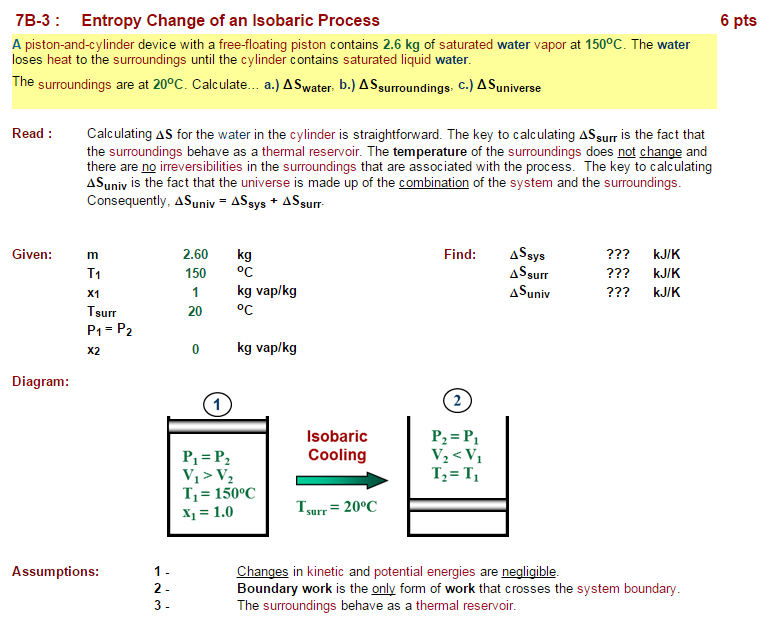
Isothermal and Isobaric Processes
- Isothermal entropy change occurs when a system undergoes a process at constant temperature
- For an ideal gas, the isothermal entropy change is given by , where is the number of moles, is the gas constant, and and are the initial and final volumes
- Isobaric entropy change occurs when a system undergoes a process at constant pressure
- For an ideal gas, the isobaric entropy change is given by , where is the molar heat capacity at constant pressure and and are the initial and final temperatures
Isochoric and Adiabatic Processes
- Isochoric entropy change occurs when a system undergoes a process at constant volume
- For an ideal gas, the isochoric entropy change is given by , where is the molar heat capacity at constant volume
- Adiabatic entropy change occurs when a system undergoes a process with no heat transfer to or from the surroundings
- For an adiabatic process, the entropy change is always zero:
- Examples include adiabatic compression or expansion of a gas in an insulated cylinder
Graphical Representation
Temperature-Entropy (T-s) Diagram
- A T-s diagram is a graphical representation of a thermodynamic process, with temperature on the y-axis and entropy on the x-axis
- Isothermal processes appear as horizontal lines on a T-s diagram, as temperature remains constant
- Isobaric processes have a positive slope on a T-s diagram, as both temperature and entropy increase
- Isochoric processes have a positive slope on a T-s diagram, similar to isobaric processes
- Adiabatic processes appear as vertical lines on a T-s diagram, as entropy remains constant with no heat transfer
- The area under the curve on a T-s diagram represents the heat transfer during the process: