Polymer nomenclature is a crucial aspect of Polymer Chemistry, providing systems to name and classify polymeric materials. From source-based to structure-based approaches, these naming conventions enable clear communication about polymer structures and properties in both academic and industrial settings.
Understanding polymer nomenclature is essential for identifying and describing various polymer types, from simple homopolymers to complex copolymers and blends. This knowledge forms the foundation for effectively communicating about polymer synthesis, characterization, and applications in the field of Polymer Chemistry.
Types of polymer nomenclature
- Polymer nomenclature encompasses various systems used to name and classify polymeric materials in the field of Polymer Chemistry
- Understanding different nomenclature types enables clear communication and identification of polymer structures and properties
- Nomenclature systems range from source-based to structure-based approaches, each serving specific purposes in polymer science
Source-based vs structure-based nomenclature
- Source-based nomenclature derives polymer names from their monomer origins
- Structure-based nomenclature focuses on the polymer's repeating unit and overall molecular structure
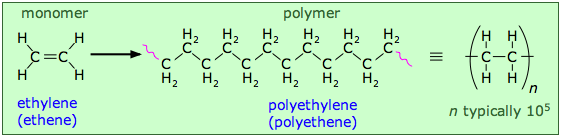
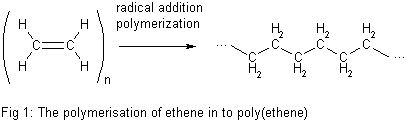
- Source-based names often used in industry for simplicity (polyethylene)
- Structure-based names provide more detailed information about polymer composition and arrangement
IUPAC nomenclature for polymers
- International Union of Pure and Applied Chemistry (IUPAC) establishes standardized naming conventions for polymers
- IUPAC system prioritizes clarity and unambiguity in polymer naming
- Incorporates both source-based and structure-based approaches depending on polymer complexity
- Utilizes prefixes and suffixes to denote specific structural features (poly, -ene, -amide)
Trade names and common names
- Trade names represent proprietary or branded polymer products (Kevlar, Teflon)
- Common names arise from widespread usage in industry or academia (nylon, plexiglass)
- Often simpler and more recognizable than systematic names but may lack specificity
- Important to distinguish between trade names, common names, and systematic nomenclature in scientific communication
Source-based nomenclature
- Source-based nomenclature derives polymer names from their constituent monomers
- This approach simplifies naming for many common polymers and is widely used in industry
- Understanding source-based naming is crucial for relating polymer structures to their starting materials
Monomer-derived names
- Polymer names directly reflect the monomer used in synthesis
- Prefix "poly" added to the monomer name (polystyrene from styrene monomer)
- Retains information about the polymer's chemical origin
- Useful for quickly identifying the basic composition of a polymer
Prefix and suffix conventions
- Prefixes indicate the number of carbon atoms in the monomer (poly(hexene))
- Suffixes denote functional groups present in the polymer (-ol for alcohols, -amide for amides)
- Combination of prefixes and suffixes provides information about both structure and composition
- Parentheses used to separate monomer name from poly prefix when necessary
Examples of source-based names
- Polyethylene derived from ethylene monomer
- Poly(vinyl chloride) or PVC from vinyl chloride
- Polypropylene from propylene monomer
- Poly(methyl methacrylate) or PMMA from methyl methacrylate monomer
Structure-based nomenclature
- Structure-based nomenclature focuses on the polymer's repeating unit and overall molecular arrangement
- This system provides more detailed information about the polymer's chemical structure
- Essential for accurately describing complex polymers with multiple components or unique architectures
Constitutional repeating unit (CRU)
- Represents the smallest repeating structural unit in the polymer chain
- Identified by analyzing the polymer's chemical structure
- Forms the basis for structure-based naming in IUPAC nomenclature
- Can be represented using line structures or condensed formulas
Seniority rules for CRU selection
- Determine the main chain based on the longest continuous sequence of atoms
- Prioritize heteroatoms over carbon atoms in main chain selection
- Consider multiple bonds and cyclic structures when identifying the main chain
- Apply IUPAC organic nomenclature rules to name the selected CRU
Naming substituents and end groups
- Identify and name side groups attached to the main chain
- Use prefixes to indicate the position and type of substituents
- Describe end groups using appropriate prefixes or suffixes
- Include stereochemical information when relevant (cis, trans, R, S configurations)
Copolymer nomenclature
- Copolymer nomenclature addresses the naming of polymers composed of two or more different monomers
- This system is crucial for describing the diverse range of copolymer structures in Polymer Chemistry
- Understanding copolymer nomenclature enables precise communication about complex polymer compositions
Block copolymer naming
- Names indicate distinct segments of different monomer units
- Use hyphens to separate block names (polystyrene-block-polybutadiene)
- Specify block sequence and length when known
- Incorporate abbreviations for clarity in complex structures (PS-b-PB)
Random copolymer naming
- Denotes copolymers with randomly distributed monomer units
- Use "co" or "random" to indicate random distribution (poly(styrene-co-butadiene))
- List monomers in alphabetical order or by molar ratio
- Include composition information when available (poly(styrene-random-butadiene) 70:30)
Alternating copolymer naming
- Describes copolymers with regularly alternating monomer units
- Use "alt" to indicate alternating structure (poly(styrene-alt-maleic anhydride))
- List monomers in the order they appear in the polymer chain
- Specify any deviations from perfect alternation if known
Abbreviations in polymer nomenclature
- Abbreviations play a crucial role in simplifying complex polymer names
- Understanding common abbreviations is essential for efficient communication in Polymer Chemistry
- Abbreviations can vary between academic and industrial contexts
Common polymer abbreviations
- PE for polyethylene
- PP for polypropylene
- PS for polystyrene
- PVC for poly(vinyl chloride)
- PTFE for polytetrafluoroethylene (Teflon)
Naming rules for abbreviations
- Use capital letters for each word in the full polymer name
- Omit articles, prepositions, and conjunctions in abbreviations
- Include numbers or Greek letters when necessary (PET for poly(ethylene terephthalate))
- Maintain consistency in abbreviation usage within a document or field
Industry-specific abbreviations
- ABS for acrylonitrile butadiene styrene
- PEEK for polyether ether ketone
- UHMWPE for ultra-high molecular weight polyethylene
- TPU for thermoplastic polyurethane
Naming polymer architectures
- Polymer architecture nomenclature describes the overall shape and arrangement of polymer chains
- This aspect of naming is crucial for understanding structure-property relationships in Polymer Chemistry
- Different architectures can significantly impact a polymer's physical and chemical properties
Linear vs branched polymers
- Linear polymers named without specific architectural designations
- Branched polymers include terms like "branched" or "hyperbranched" in their names
- Specify branch density or distribution when known (lightly branched, highly branched)
- Include information about branch length or composition for complex structures
Crosslinked polymer nomenclature
- Use "crosslinked" or "network" to indicate three-dimensional structures
- Specify crosslinking agent or method when relevant (sulfur-crosslinked, radiation-crosslinked)
- Include crosslink density information if available (lightly crosslinked, densely crosslinked)
- Distinguish between physical and chemical crosslinking in nomenclature
Dendrimer and star polymer naming
- Dendrimers named with "dendrimer" suffix and generation number (G4-PAMAM dendrimer)
- Star polymers include "star" in the name and specify arm number and composition
- Describe core structure for both dendrimers and star polymers when relevant
- Include information about end group functionality for these complex architectures
Stereochemical nomenclature
- Stereochemical nomenclature in polymers addresses the spatial arrangement of atoms along the polymer chain
- This aspect of naming is crucial for understanding and predicting polymer properties in Polymer Chemistry
- Stereochemistry can significantly impact a polymer's physical, mechanical, and optical characteristics
Tacticity in polymer naming
- Isotactic polymers have all substituents on the same side of the polymer backbone
- Syndiotactic polymers have alternating substituents on opposite sides of the backbone
- Atactic polymers have randomly arranged substituents
- Include tacticity information in parentheses after the polymer name (polypropylene (isotactic))
Cis-trans isomerism nomenclature
- Specify cis or trans configuration for polymers with double bonds in the backbone
- Use prefixes to indicate the predominant isomer (cis-1,4-polyisoprene)
- Include percentages of cis and trans content when known (polybutadiene (60% cis, 40% trans))
- Consider the impact of cis-trans isomerism on polymer properties (natural vs synthetic rubber)
Chiral polymer nomenclature
- Indicate the presence of chiral centers using R or S designations
- Specify overall chirality of the polymer if applicable (right-handed helical polypeptide)
- Include information about optical activity (dextrorotatory, levorotatory)
- Consider the implications of chirality on polymer applications (chiral separation media)
Nomenclature for polymer blends
- Polymer blend nomenclature addresses the naming of mixtures of two or more distinct polymers
- Understanding blend nomenclature is crucial for describing complex material systems in Polymer Chemistry
- Proper naming of blends enables clear communication about composite materials and their properties
Binary blend naming conventions
- List component polymers separated by a forward slash (PS/PMMA blend)
- Include weight or volume ratios when known (PE/PP 70:30 blend)
- Specify whether the blend is miscible or immiscible if relevant
- Use full names or abbreviations consistently within a document
Multicomponent blend nomenclature
- Extend binary blend conventions to include all components (PA/PE/PP blend)
- List components in order of decreasing concentration or alphabetically
- Include ratios for all components when possible (ABS/PC/PMMA 50:30:20 blend)
- Consider using tabular formats for complex blend compositions
Interpenetrating network (IPN) naming
- Distinguish between full IPNs and semi-IPNs in nomenclature
- Specify component polymers and their relative amounts (Polyurethane/Polyacrylic acid IPN)
- Include information about the synthesis method if relevant (simultaneous IPN, sequential IPN)
- Consider the impact of IPN structure on material properties (enhanced mechanical strength, controlled drug release)
International standards
- International standards in polymer nomenclature ensure consistency and clarity in scientific communication
- Understanding these standards is essential for Polymer Chemistry students to effectively engage with global research
- Different organizations contribute to the development and maintenance of polymer naming conventions
ISO polymer naming guidelines
- International Organization for Standardization (ISO) provides guidelines for polymer nomenclature
- ISO standards cover various aspects of polymer naming and classification
- Include specific standards for abbreviated terms (ISO 1043) and plastics (ISO 472)
- Regularly updated to accommodate new polymer types and industry developments
ASTM nomenclature standards
- American Society for Testing and Materials (ASTM) develops standards for polymer nomenclature
- ASTM D1600 provides standard terminology for abbreviated terms in polymer science
- Includes guidelines for naming test methods and material specifications
- Widely used in North American polymer industry and research
Regional naming variations
- European Committee for Standardization (CEN) develops polymer nomenclature standards for Europe
- Japanese Industrial Standards (JIS) provide naming conventions for polymers in Japan
- Consider regional variations when interpreting polymer names in international contexts
- Strive for consistency with IUPAC nomenclature to minimize confusion across regions
Nomenclature in polymer characterization
- Nomenclature in polymer characterization is crucial for accurately describing analytical techniques and results
- Understanding this specialized vocabulary is essential for Polymer Chemistry students to interpret and communicate research findings
- Different characterization methods often have their own specific terminology and abbreviations
NMR spectroscopy terminology
- Describe polymer microstructure using terms like tacticity, sequence distribution, and end group analysis
- Specify NMR nuclei used in the analysis (1H NMR, 13C NMR, 19F NMR)
- Include information about solvent and temperature conditions
- Use chemical shift (δ) values to report peak positions in parts per million (ppm)
GPC/SEC naming conventions
- Gel Permeation Chromatography (GPC) and Size Exclusion Chromatography (SEC) used interchangeably
- Report molecular weight averages using standardized abbreviations (Mn, Mw, Mz)
- Specify polydispersity index (PDI) or dispersity (Ð) to describe molecular weight distribution
- Include information about calibration standards and eluent used in the analysis
Thermal analysis nomenclature
- Differential Scanning Calorimetry (DSC) used to report glass transition temperature (Tg), melting temperature (Tm), and crystallization temperature (Tc)
- Thermogravimetric Analysis (TGA) describes decomposition temperature (Td) and char yield
- Dynamic Mechanical Analysis (DMA) reports storage modulus (E'), loss modulus (E"), and tan δ
- Specify heating/cooling rates and atmosphere conditions for all thermal analyses