Electrochemical cells and standard reduction potentials are key to understanding redox reactions. These concepts explain how we can harness electron transfer to generate electricity or drive chemical changes. They're the backbone of batteries, fuel cells, and many industrial processes.
Knowing standard reduction potentials helps predict which reactions will happen spontaneously. This info is crucial for designing efficient energy storage systems and understanding corrosion. It's all about the flow of electrons and the energy changes that come with it.
Redox Reactions
Oxidation and Reduction Processes
- Oxidation involves loss of electrons from a chemical species
- Increases the oxidation state of an element
- Occurs at the anode in electrochemical cells
- Reduction entails gain of electrons by a chemical species
- Decreases the oxidation state of an element
- Takes place at the cathode in electrochemical cells
- Redox reactions couple oxidation and reduction processes
- One species undergoes oxidation while another undergoes reduction
- Electron transfer occurs between the two species
- Half-reactions split redox reactions into separate oxidation and reduction steps
- Useful for balancing complex redox equations
- Aid in understanding the electron flow in electrochemical cells
Oxidation States and Electron Transfer
- Oxidation states represent the degree of oxidation of an atom in a compound
- Range from -4 to +7 for most elements
- Determined by electronegativity differences and bonding
- Electron transfer drives redox reactions
- Electrons move from the reducing agent to the oxidizing agent
- The number of electrons lost equals the number of electrons gained
- Redox reactions occur in various contexts
- Combustion reactions (burning of fuels)
- Corrosion of metals (rusting of iron)
- Biological processes (photosynthesis, cellular respiration)
Electrochemical Cells
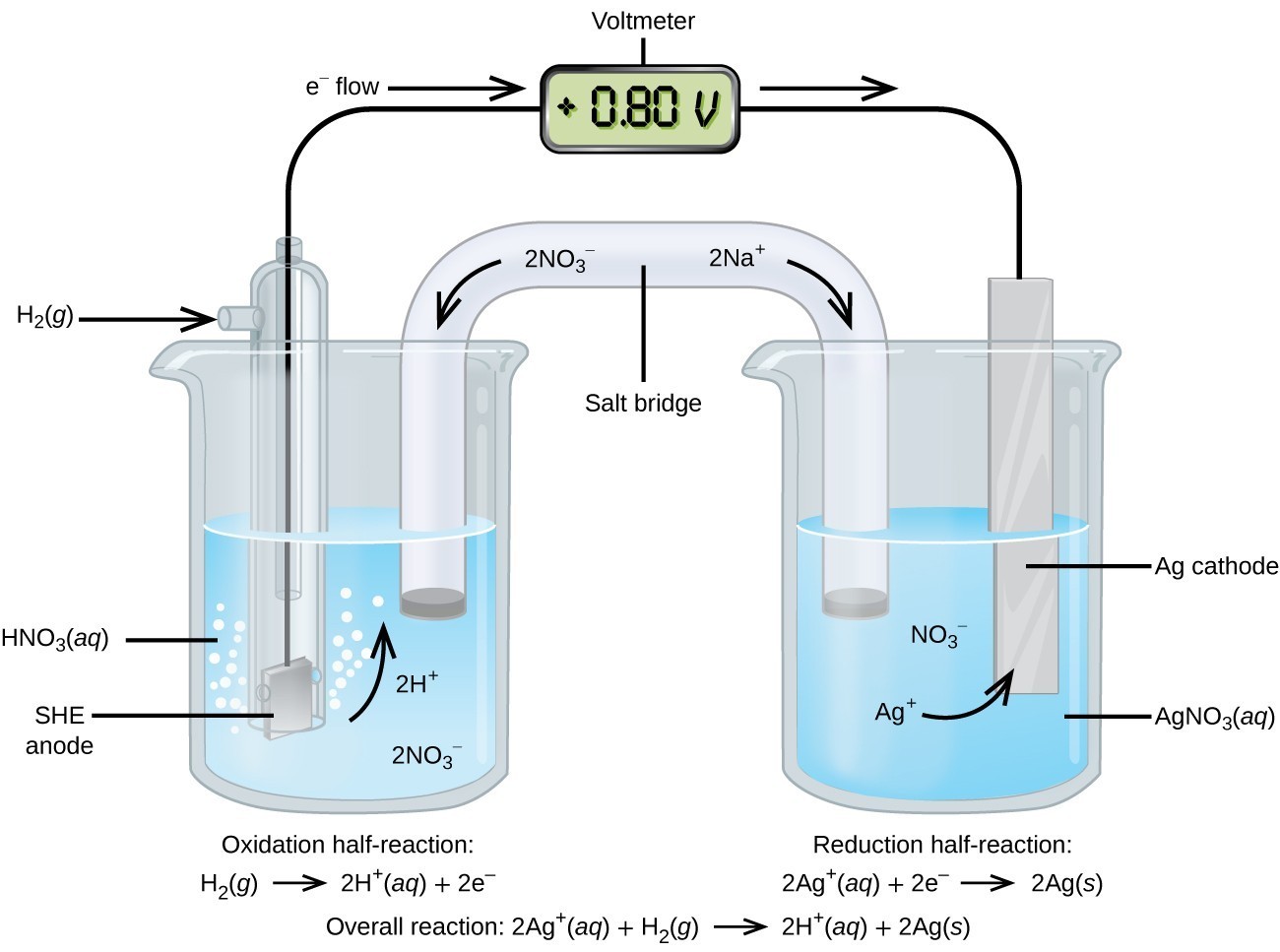
Components and Structure
- Electrochemical cells convert chemical energy into electrical energy or vice versa
- Consist of two half-cells connected by a salt bridge or porous barrier
- Each half-cell contains an electrode immersed in an electrolyte solution
- Anode functions as the site of oxidation
- Negative electrode in galvanic cells
- Positive electrode in electrolytic cells
- Releases electrons into the external circuit
- Cathode serves as the site of reduction
- Positive electrode in galvanic cells
- Negative electrode in electrolytic cells
- Accepts electrons from the external circuit
- Salt bridge maintains electrical neutrality
- Contains a concentrated electrolyte solution
- Allows ion flow between half-cells
- Prevents mixing of different electrolyte solutions
Types of Electrochemical Cells
- Galvanic cells produce electricity from spontaneous redox reactions
- Also known as voltaic cells
- Used in batteries and fuel cells
- Electrons flow from anode to cathode through an external circuit
- Electrolytic cells use electricity to drive non-spontaneous redox reactions
- Employed in electroplating and electrolysis processes
- Require an external power source to push electrons from anode to cathode
- Often used for metal purification or production of chemicals
Standard Reduction Potentials

Standard Hydrogen Electrode and Reference
- Standard hydrogen electrode (SHE) serves as a universal reference
- Assigned a standard reduction potential of 0.00 V
- Consists of a platinum electrode in contact with 1 M H+ and H2 gas at 1 atm
- Used to measure reduction potentials of other half-reactions
- Standard reduction potential quantifies the tendency of a species to be reduced
- Measured in volts (V) under standard conditions (1 M, 1 atm, 25°C)
- More positive values indicate a greater tendency to be reduced
- Tabulated in electrochemical series for easy reference
Electrochemical Series and Predictions
- Electrochemical series ranks elements and compounds by their standard reduction potentials
- Arranged from most negative (least likely to be reduced) to most positive (most likely to be reduced)
- Helps predict the direction of redox reactions and relative strengths of oxidizing and reducing agents
- Standard reduction potentials enable various predictions
- Spontaneity of redox reactions
- Strength of oxidizing and reducing agents
- Feasibility of metal displacement reactions
- Calculating cell potentials using standard reduction potentials
- Positive cell potential indicates a spontaneous reaction
Cell Potential and Nernst Equation
Cell Potential and Gibbs Free Energy
- Cell potential represents the voltage difference between two electrodes
- Measured in volts (V)
- Indicates the driving force for electron flow in an electrochemical cell
- Related to the Gibbs free energy change of the reaction
- Relationship between cell potential and Gibbs free energy
- n = number of electrons transferred
- F = Faraday's constant (96,485 C/mol)
- Negative ΔG° indicates a spontaneous reaction
Nernst Equation and Non-Standard Conditions
- Nernst equation relates cell potential to reaction conditions
- Accounts for non-standard concentrations and temperatures
- R = gas constant, T = temperature in Kelvin, Q = reaction quotient
- Applications of the Nernst equation
- Calculating cell potentials under non-standard conditions
- Determining equilibrium constants for redox reactions
- Predicting the direction of electron flow in concentration cells
- Concentration cells utilize the Nernst equation
- Generate electrical energy from concentration differences
- Have identical half-reactions but different concentrations
- Electron flow occurs from the dilute to the concentrated solution