Amperometric methods measure current at a fixed potential to determine analyte concentration. These techniques rely on mass transport and electrode surface characteristics for accurate results. They're widely used in flow injection analysis and biosensors.
Amperometry offers high sensitivity, fast response times, and compatibility with biological samples. However, it can face interference from other electroactive species and electrode fouling. Understanding these principles is crucial for effective application in electrochemical analysis.
Amperometric Methods: Principles and Applications
Principles of amperometric methods
- Measure current at a fixed potential held constant while monitoring current over time
- Current is proportional to the concentration of the analyte being measured
- Differ from voltammetry which sweeps potential over a range and records resulting current to provide information about redox processes at the electrode surface
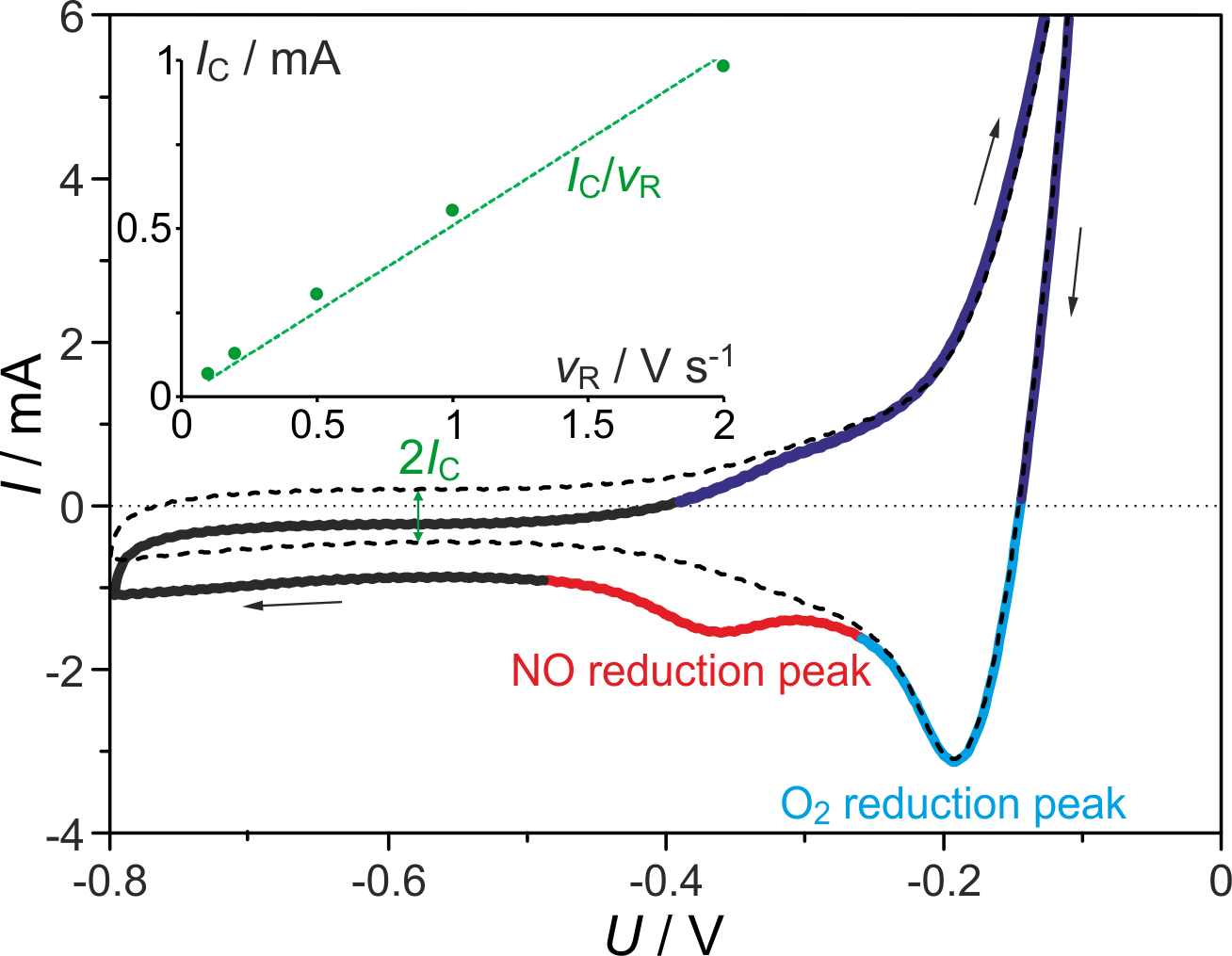
Factors in amperometric response
- Mass transport plays crucial role, primarily through diffusion of analyte molecules from bulk solution to electrode surface
- Diffusion rate depends on concentration gradient and diffusion coefficient
- Convection from stirring or solution flow can also enhance mass transport and maintain constant analyte concentration near electrode
- Electrode surface characteristics influence response
- Surface area affects current magnitude (larger area = higher current)
- Cleanliness and pretreatment important to ensure reproducible results by removing contaminants or oxide layers that hinder electron transfer
- Material and modification can enhance selectivity and sensitivity due to varying catalytic properties, electron transfer kinetics, or surface modification with enzymes, polymers, nanoparticles
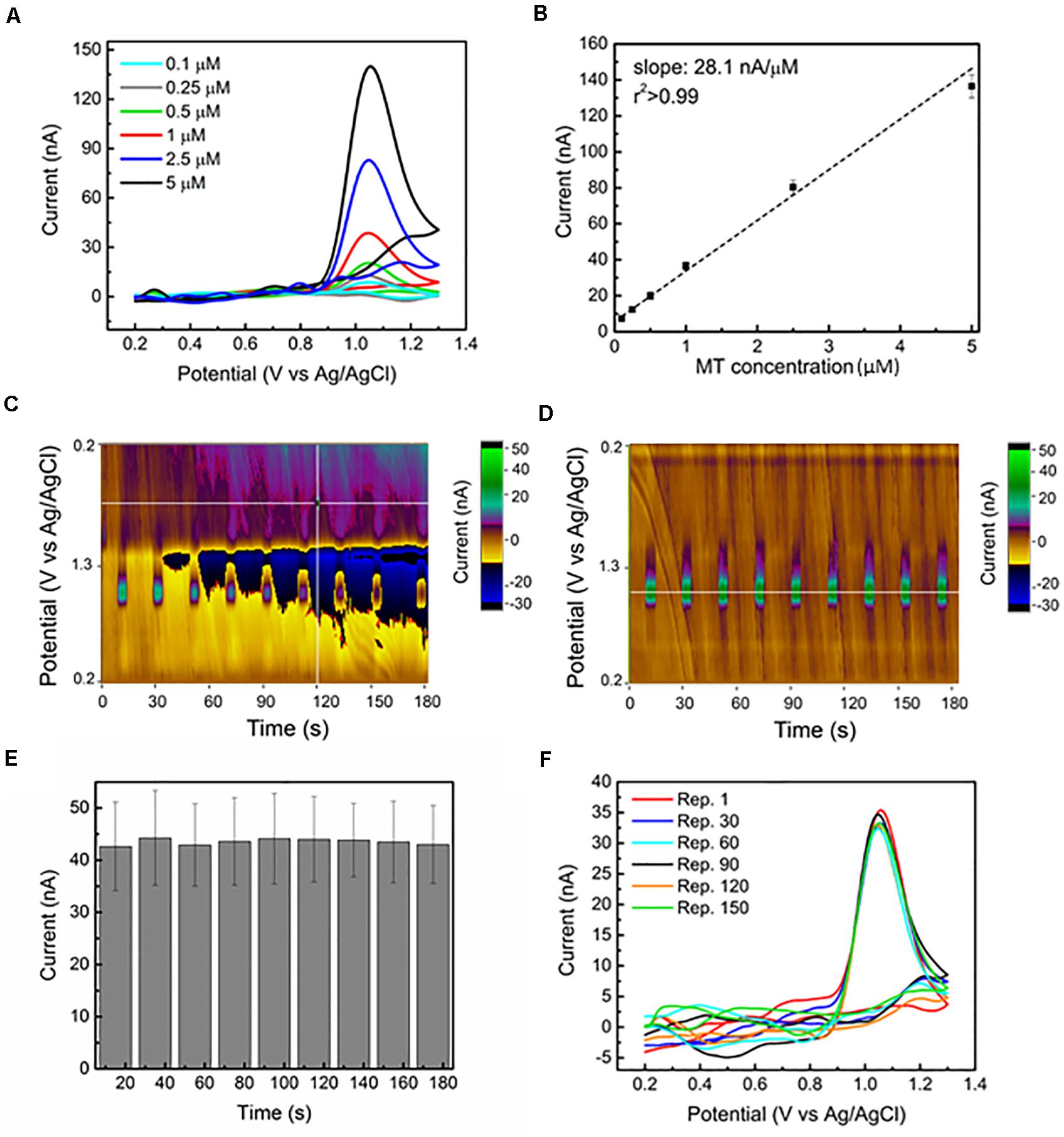
Applications of amperometric detection
- Flow injection analysis (FIA) combines with flow system
- Analyte injected into continuous carrier solution flow
- Sample plug reaches detector and generates transient current signal
- Peak height or area is proportional to analyte concentration
- Allows rapid, automated analysis of multiple samples
- Biosensors utilize for detecting biological analytes
- Enzymes or antibodies immobilized on electrode surface interact with analyte to produce electrochemical signal
- Glucose biosensors a common example
- Glucose oxidase catalyzes glucose oxidation to generate hydrogen peroxide
- Hydrogen peroxide detected amperometrically to measure glucose concentration
- Offer high selectivity, sensitivity, ability to analyze complex biological samples
Amperometry vs other electrochemical techniques
- Advantages:
- High sensitivity and low detection limits
- Fast response time and real-time monitoring
- Can measure both oxidizable and reducible species
- Suitable for flow systems and miniaturization
- Compatible with biological samples and in vivo measurements
- Limitations:
- Interference from other electroactive species in sample matrix
- Electrode surface fouling over time requires regular cleaning or replacement
- Less selective than techniques like potentiometry with ion-selective electrodes
- Difficult to directly measure non-electroactive analytes
- Sensitive to environmental factors (temperature, pH)