Self-assembly in colloidal systems is a fascinating process where particles organize into ordered structures without external control. This spontaneous organization is driven by interactions between particles and their environment, resulting in complex architectures with unique properties.
Understanding self-assembly principles is crucial for designing advanced materials and technologies. From drug delivery systems to nanomaterials, self-assembly enables the creation of structures with tailored functions, opening up exciting possibilities in various fields of science and engineering.
Fundamentals of self-assembly
- Self-assembly is a critical process in colloidal systems where individual components spontaneously organize into ordered structures
- Understanding the principles of self-assembly is essential for designing and controlling the formation of complex colloidal architectures
- Self-assembly processes are governed by a delicate balance of thermodynamic and kinetic factors
Definition of self-assembly
- Self-assembly refers to the spontaneous organization of individual components into ordered structures without external intervention
- Occurs through non-covalent interactions between the components (hydrogen bonding, hydrophobic interactions)
- Results in the formation of thermodynamically stable structures at equilibrium
Thermodynamic considerations
- Self-assembly is driven by the minimization of the system's free energy
- Involves a balance between enthalpy and entropy contributions
- Enthalpy: favorable interactions between components (hydrogen bonding, van der Waals forces)
- Entropy: increased disorder of the system upon assembly (release of solvent molecules, conformational changes)
- The final self-assembled structure represents the state with the lowest free energy
Kinetic factors in self-assembly
- Kinetic factors determine the pathway and rate of self-assembly
- Influenced by the activation energy barriers for assembly and disassembly processes
- Factors affecting kinetics include:
- Diffusion rates of components
- Collision frequency and orientation
- Presence of intermediates or metastable states
- Kinetic control can be used to trap non-equilibrium structures or direct the assembly towards specific morphologies
Types of self-assembled structures
- Self-assembly in colloidal systems can lead to the formation of various ordered structures with distinct morphologies and properties
- The type of self-assembled structure depends on the characteristics of the building blocks and the environmental conditions
- Common self-assembled structures include micelles, vesicles, bilayers, and liquid crystals
Micelles and vesicles
- Micelles are spherical aggregates formed by amphiphilic molecules in aqueous solutions
- Hydrophobic tails orient towards the interior, while hydrophilic heads face the aqueous environment
- Formed above the critical micelle concentration (CMC)
- Vesicles are closed bilayer structures encapsulating an aqueous compartment
- Can be unilamellar (single bilayer) or multilamellar (multiple concentric bilayers)
- Used for encapsulation and delivery of hydrophilic molecules (drugs, enzymes)
Bilayers and membranes
- Bilayers are two-dimensional sheet-like structures formed by the self-assembly of amphiphilic molecules
- Consist of two opposing monolayers with hydrophobic tails facing each other and hydrophilic heads exposed to the aqueous environment
- Form the basis of biological membranes (cell membranes, organelle membranes)
- Can be used to create artificial membranes for separation, sensing, or catalysis applications
Liquid crystals
- Liquid crystals are mesophases that exhibit long-range orientational order but lack long-range positional order
- Formed by anisotropic molecules (rod-like or disc-like) that align along a preferred direction
- Exhibit unique optical and electrical properties due to their ordered structure
- Types of liquid crystals include nematic, smectic, and cholesteric phases
- Find applications in display technologies (LCDs), sensors, and responsive materials
Driving forces for self-assembly
- Self-assembly in colloidal systems is driven by various non-covalent interactions between the building blocks
- These interactions determine the stability, structure, and properties of the self-assembled aggregates
- The main driving forces include hydrophobic interactions, hydrogen bonding, electrostatic interactions, and van der Waals forces
Hydrophobic interactions
- Hydrophobic interactions are the primary driving force for the self-assembly of amphiphilic molecules in aqueous solutions
- Arise from the tendency of hydrophobic moieties to minimize their contact with water molecules
- Lead to the aggregation of hydrophobic tails in the interior of micelles or bilayers, while hydrophilic heads remain exposed to water
- Strength of hydrophobic interactions increases with the size and hydrophobicity of the molecules
Hydrogen bonding
- Hydrogen bonding is an attractive interaction between a hydrogen atom bonded to an electronegative atom (donor) and another electronegative atom (acceptor)
- Plays a crucial role in the self-assembly of molecules with complementary hydrogen bonding sites (nucleic acids, peptides)
- Directs the formation of specific secondary structures (α-helices, β-sheets) and supramolecular architectures
- Contributes to the stability and selectivity of self-assembled structures
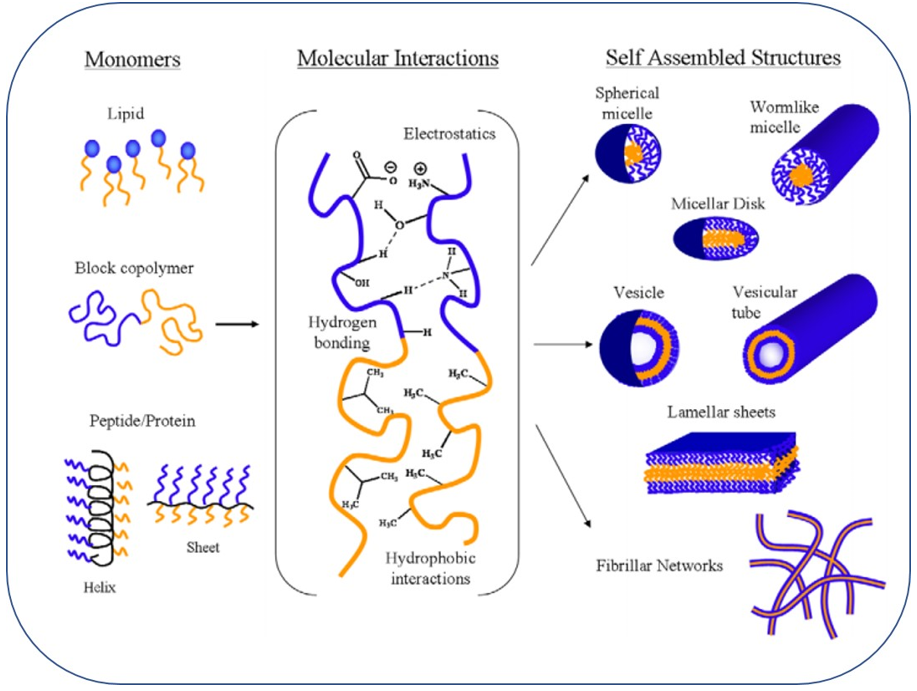
Electrostatic interactions
- Electrostatic interactions occur between charged or polarizable molecules
- Can be attractive (opposite charges) or repulsive (like charges)
- Influence the self-assembly of ionic surfactants, polyelectrolytes, and charged nanoparticles
- Screened by the presence of counterions in solution, with the screening length determined by the ionic strength
- Can be tuned by adjusting the pH or ionic strength of the medium
Van der Waals forces
- Van der Waals forces are weak, short-range attractive interactions between molecules arising from induced dipoles
- Include dispersion forces (London forces), dipole-dipole interactions (Keesom forces), and dipole-induced dipole interactions (Debye forces)
- Contribute to the overall stability of self-assembled structures, particularly in the absence of stronger interactions
- Become significant when the molecules are in close proximity, such as in tightly packed structures or at high concentrations
Factors influencing self-assembly
- The self-assembly process in colloidal systems is influenced by various factors related to the properties of the building blocks and the environmental conditions
- These factors determine the final structure, size, and properties of the self-assembled aggregates
- Key factors include particle size and shape, surface chemistry and functionalization, solvent properties, temperature, and pressure
Particle size and shape
- The size and shape of the building blocks play a critical role in their self-assembly behavior
- Smaller particles have a higher surface area-to-volume ratio, which can enhance the influence of surface interactions
- Anisotropic particles (rods, discs, polyhedra) exhibit shape-dependent packing and orientation in self-assembled structures
- Particle size distribution affects the uniformity and reproducibility of the self-assembly process
Surface chemistry and functionalization
- The surface chemistry of the building blocks determines their interactions with each other and the surrounding medium
- Functionalization with specific chemical groups (charged moieties, hydrophobic chains) can direct the self-assembly process
- Surface modification can be used to control the hydrophobicity/hydrophilicity balance, charge density, or specific binding sites
- Responsive surface functionalization (pH-sensitive, thermoresponsive) enables the creation of stimuli-responsive self-assembled structures
Solvent properties
- The properties of the solvent, such as polarity, dielectric constant, and viscosity, influence the self-assembly process
- Solvent-particle interactions determine the solvation and stability of the building blocks
- Solvent quality affects the conformation and interactions of polymeric or macromolecular building blocks
- Solvent mixtures can be used to tune the self-assembly behavior by altering the solvophobic/solvophilic balance
Temperature and pressure
- Temperature and pressure are important thermodynamic parameters that affect self-assembly
- Increasing temperature typically enhances the thermal motion of molecules, which can disrupt or reorganize self-assembled structures
- Lower temperatures favor the formation of more ordered and stable structures
- Pressure can influence the packing and phase behavior of self-assembled systems, particularly in liquid crystalline or polymeric materials
- Varying temperature or pressure can induce phase transitions or trigger the formation of different self-assembled morphologies
Characterization techniques
- Characterizing self-assembled structures in colloidal systems requires a combination of microscopic, scattering, and spectroscopic techniques
- These techniques provide information about the size, shape, internal structure, and chemical composition of the self-assembled aggregates
- Commonly used characterization methods include microscopy, scattering, and spectroscopy
Microscopy methods
- Microscopy techniques allow direct visualization of self-assembled structures with high spatial resolution
- Transmission electron microscopy (TEM) provides 2D projections of the sample with nanoscale resolution
- Requires sample staining or cryogenic preparation for improved contrast
- Scanning electron microscopy (SEM) offers surface topography information with a larger depth of field
- Atomic force microscopy (AFM) enables 3D imaging of surfaces with nanometer resolution and can probe mechanical properties
- Fluorescence microscopy allows the visualization of labeled components or specific interactions within self-assembled structures
Scattering techniques
- Scattering techniques provide ensemble-averaged information about the size, shape, and internal structure of self-assembled systems
- Small-angle X-ray scattering (SAXS) and small-angle neutron scattering (SANS) are widely used for characterizing colloidal structures
- Provide information about the size distribution, shape, and internal organization of the scattering objects
- Dynamic light scattering (DLS) measures the hydrodynamic size and size distribution of particles or aggregates in solution
- Static light scattering (SLS) can determine the molar mass, radius of gyration, and second virial coefficient of macromolecular assemblies

Spectroscopic analysis
- Spectroscopic techniques probe the chemical composition, interactions, and dynamics of self-assembled systems
- Fourier-transform infrared (FTIR) spectroscopy identifies functional groups and intermolecular interactions within the assemblies
- Nuclear magnetic resonance (NMR) spectroscopy provides detailed information about the molecular structure, dynamics, and local environment of the components
- Circular dichroism (CD) spectroscopy is sensitive to the secondary structure and chirality of self-assembled biomolecules (peptides, proteins)
- Fluorescence spectroscopy can monitor the local environment, conformational changes, or intermolecular interactions using fluorescent probes or labels
Applications of self-assembly
- Self-assembly in colloidal systems has found numerous applications in various fields, including drug delivery, nanomaterials synthesis, biosensing, and self-healing materials
- The unique properties and functionalities of self-assembled structures make them attractive for diverse technological and biomedical applications
- Some key application areas are highlighted below
Drug delivery systems
- Self-assembled structures, such as micelles, vesicles, and liposomes, can be used as carriers for controlled drug delivery
- Hydrophobic drugs can be encapsulated within the hydrophobic core of micelles, improving their solubility and bioavailability
- Vesicles and liposomes can encapsulate both hydrophobic and hydrophilic drugs, protecting them from premature degradation
- Stimuli-responsive self-assembled carriers (pH-sensitive, thermoresponsive) enable targeted drug release at specific sites or conditions
Nanomaterials synthesis
- Self-assembly provides a bottom-up approach for the synthesis of functional nanomaterials with well-defined structures and properties
- Block copolymers can self-assemble into ordered nanostructures (spheres, cylinders, lamellae) with tunable sizes and periodicities
- Nanoparticle superlattices can be formed by the self-assembly of inorganic nanoparticles, leading to novel optical, electronic, or magnetic properties
- Biomolecular self-assembly (peptides, DNA) can be harnessed for the fabrication of bio-inspired nanomaterials with specific functions
Biosensors and diagnostics
- Self-assembled monolayers (SAMs) can be used as functional interfaces for biosensing and diagnostic applications
- SAMs can be functionalized with specific recognition elements (antibodies, aptamers) for the selective detection of target analytes
- Self-assembled nanostructures with high surface area and tunable properties can enhance the sensitivity and specificity of biosensors
- Responsive self-assembled systems can be designed to produce measurable signals (optical, electrical) upon binding of the target molecules
Self-healing materials
- Self-assembly can be exploited to create materials with self-healing capabilities, mimicking biological systems
- Supramolecular polymers formed by reversible non-covalent interactions can undergo dynamic exchange and reorganization, enabling self-repair
- Self-assembled networks with reversible crosslinks (hydrogen bonds, metal-ligand coordination) can autonomously heal upon damage
- Stimuli-responsive self-healing materials can be triggered by external stimuli (light, heat, pH) to initiate the repair process
Challenges and limitations
- Despite the significant progress in understanding and applying self-assembly in colloidal systems, several challenges and limitations still need to be addressed
- These challenges relate to the control over assembly kinetics, achieving monodispersity, scalability, and stability of the self-assembled structures
- Addressing these challenges is crucial for the reliable and efficient implementation of self-assembly in practical applications
Controlling assembly kinetics
- Precise control over the kinetics of self-assembly is essential for obtaining well-defined and reproducible structures
- The rate of assembly and disassembly processes can be influenced by various factors, such as temperature, concentration, and additives
- Balancing the kinetics of nucleation and growth stages is critical for achieving uniform and monodisperse self-assembled structures
- Strategies for controlling assembly kinetics include seeded growth, step-wise assembly, and the use of external fields or templates
Achieving monodispersity
- Monodispersity refers to the uniformity in size, shape, and composition of the self-assembled structures
- Polydisperse systems exhibit a distribution of sizes or morphologies, which can affect their properties and performance
- Achieving high monodispersity is challenging due to the inherent variability in the self-assembly process and the polydispersity of the building blocks
- Purification techniques, such as size-selective precipitation or chromatography, can be employed to narrow the size distribution of self-assembled structures
Scaling up production
- Scaling up the production of self-assembled structures from the laboratory scale to industrial levels presents several challenges
- Maintaining the uniformity and reproducibility of the self-assembly process at larger scales can be difficult due to variations in mixing, heat transfer, and mass transport
- The cost and availability of the building blocks, as well as the efficiency of the self-assembly process, need to be considered for commercial viability
- Process intensification strategies, such as continuous flow synthesis or microfluidic platforms, can aid in the scalable production of self-assembled structures
Stability of self-assembled structures
- The stability of self-assembled structures is crucial for their long-term performance and shelf life
- Self-assembled structures are held together by non-covalent interactions, which can be sensitive to changes in environmental conditions (pH, temperature, ionic strength)
- Dissociation or reorganization of the self-assembled structures over time can lead to loss of functionality or undesired side effects
- Strategies for improving stability include covalent crosslinking, encapsulation, or the incorporation of stabilizing additives
- Designing self-assembled structures with built-in error correction mechanisms or self-healing capabilities can enhance their stability and resilience