
Valence Electrons
4 min read•december 10, 2021
Sitara H
Sitara H
What Are Valence Electrons and How to Find them
In a nutshell, a valence electron is an electron located on the outermost shell of an atom. It plays a part in many chemical reactions that the atom goes through.
Finding the Valence Electrons
To determine the number of valence electrons of an atom, we have to look at the periodic table and search for the element's position.
Main Group Elements
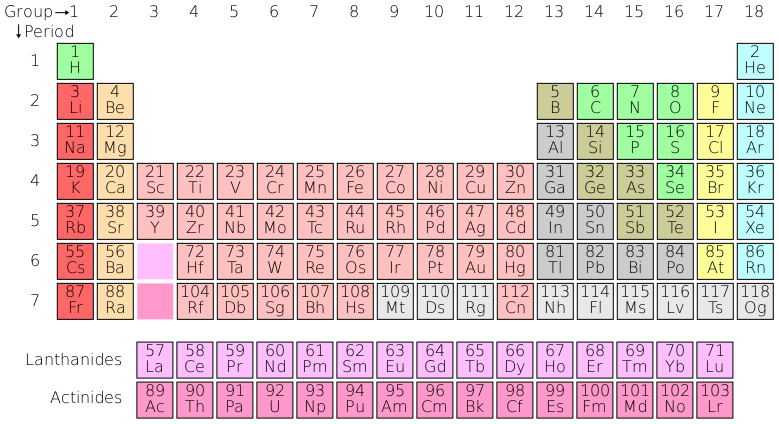
The table contains 18 columns (known as groups) and seven rows (known as periods). The transition elements form a bridge between Groups 2 and 13, with the lanthanides and actinides included.
When you look down a group, the number of valence electrons of an element remains the same, but the number of shells increases. For example, both Beryllium (Be) and Calcium (Ca) have two valence electrons, but Beryllium has 2 electron shells while Calcium has 4. When you look across a period, the number of electron shells stays the same, but the number of valence electrons increases incrementally.
💡 To figure out how many electron shells an atom has encircling its nucleus, just look at the period number! Look at the unit digit of the group number to determine the number of valence electrons for elements in that group.
Transition Elements
However, these rules only apply to main group elements in Groups 1-2 and 13-18. The rules mentioned above don't apply to the transition and inner transition elements (another name to refer to lanthanides and actinides)!
So why don't transition elements work like every other element? The answer is long and complex but boils down to orbitals-- the paths electrons take around the nucleus. They vary quite unpredictably, and therefore we can't predict ❌ the number of valence electrons for transition elements.
Reactivity
The number of valence electrons an atom has can tell you a lot about its reactivity.
Atoms with one or two extra valence electrons that form a complete shell (known as the alkali and alkaline earth metals, respectively) are highly reactive, and so are those with one or two less than a full shell (groups 17 (halogens) and 16).
In this way, you can predict 🔮 which elements will bond with each other based on how many more or fewer electrons the atoms need to have a complete valence shell.
Bonding
Every atom's goal is to have a complete valence shell with 8 electrons (or, in Hydrogen's case, 2). However, because none of the atoms have complete valence shells except for the noble gases (the left-most column on the periodic table), they all tend to bond together to complete each other's valence shells.
Covalent Bonds
In a nutshell, a covalent bond is when two atoms share a valence electron and form a bond. This type of bonding can usually be observed between nonmetals or atoms close to each other on the periodic table, with similar electronegativities (explained further below!).
These can be either single bonds (where one valence electron is shared), double bonds (where two valence electrons are shared), or triple bonds (where three valence electrons are shared).
The most common element this occurs with is Carbon (C): carbon has four valence electrons, and it is highly unfavorable for it to either lose or gain four more electrons to satisfy the octet rule. Therefore, it tends to share covalent bonds with other elements instead!
Ionic Bonds
Ionic bonding is the complete transfer of valence electrons between a metal and a nonmetal. The metal loses electrons to become a positively charged cation, while the nonmetal gains electrons to become a negatively charged anion. By losing an electron or two, the metals can satisfy the octet rule and can have a complete valence shell, while nonmetals can accept electrons to fulfill the rule as well.
In ionic bonding, the compound's net charge must be zero, with the same amount of electrons being gained and lost.
Electronegativity
Electronegativity is the tendency for an element to attract electrons towards itself. It is affected by its atomic number and the size of the atom.
The nuclear charge is important because the more positive protons an atom has (recall that the atomic number of an atom equals the protons it contains!), the more "pull" it will have on negative protons.
An electron's distance from the nucleus 📏 is also influential because the further electrons are from the nucleus, the less positive charge they experience.
The more electron shells an atom has and the higher its atomic number, the less electronegative it is. According to this, Fluorine (F) at the top right corner of the periodic table is the most electronegative atom, and Francium (Fr) 🏥 at the bottom left corner is the least electronegative.
Valence Electrons
4 min read•december 10, 2021
Sitara H
Sitara H
What Are Valence Electrons and How to Find them
In a nutshell, a valence electron is an electron located on the outermost shell of an atom. It plays a part in many chemical reactions that the atom goes through.
Finding the Valence Electrons
To determine the number of valence electrons of an atom, we have to look at the periodic table and search for the element's position.
Main Group Elements

The table contains 18 columns (known as groups) and seven rows (known as periods). The transition elements form a bridge between Groups 2 and 13, with the lanthanides and actinides included.
When you look down a group, the number of valence electrons of an element remains the same, but the number of shells increases. For example, both Beryllium (Be) and Calcium (Ca) have two valence electrons, but Beryllium has 2 electron shells while Calcium has 4. When you look across a period, the number of electron shells stays the same, but the number of valence electrons increases incrementally.
💡 To figure out how many electron shells an atom has encircling its nucleus, just look at the period number! Look at the unit digit of the group number to determine the number of valence electrons for elements in that group.
Transition Elements
However, these rules only apply to main group elements in Groups 1-2 and 13-18. The rules mentioned above don't apply to the transition and inner transition elements (another name to refer to lanthanides and actinides)!
So why don't transition elements work like every other element? The answer is long and complex but boils down to orbitals-- the paths electrons take around the nucleus. They vary quite unpredictably, and therefore we can't predict ❌ the number of valence electrons for transition elements.
Reactivity
The number of valence electrons an atom has can tell you a lot about its reactivity.
Atoms with one or two extra valence electrons that form a complete shell (known as the alkali and alkaline earth metals, respectively) are highly reactive, and so are those with one or two less than a full shell (groups 17 (halogens) and 16).
In this way, you can predict 🔮 which elements will bond with each other based on how many more or fewer electrons the atoms need to have a complete valence shell.
Bonding
Every atom's goal is to have a complete valence shell with 8 electrons (or, in Hydrogen's case, 2). However, because none of the atoms have complete valence shells except for the noble gases (the left-most column on the periodic table), they all tend to bond together to complete each other's valence shells.
Covalent Bonds
In a nutshell, a covalent bond is when two atoms share a valence electron and form a bond. This type of bonding can usually be observed between nonmetals or atoms close to each other on the periodic table, with similar electronegativities (explained further below!).
These can be either single bonds (where one valence electron is shared), double bonds (where two valence electrons are shared), or triple bonds (where three valence electrons are shared).
The most common element this occurs with is Carbon (C): carbon has four valence electrons, and it is highly unfavorable for it to either lose or gain four more electrons to satisfy the octet rule. Therefore, it tends to share covalent bonds with other elements instead!
Ionic Bonds
Ionic bonding is the complete transfer of valence electrons between a metal and a nonmetal. The metal loses electrons to become a positively charged cation, while the nonmetal gains electrons to become a negatively charged anion. By losing an electron or two, the metals can satisfy the octet rule and can have a complete valence shell, while nonmetals can accept electrons to fulfill the rule as well.
In ionic bonding, the compound's net charge must be zero, with the same amount of electrons being gained and lost.
Electronegativity
Electronegativity is the tendency for an element to attract electrons towards itself. It is affected by its atomic number and the size of the atom.
The nuclear charge is important because the more positive protons an atom has (recall that the atomic number of an atom equals the protons it contains!), the more "pull" it will have on negative protons.
An electron's distance from the nucleus 📏 is also influential because the further electrons are from the nucleus, the less positive charge they experience.
The more electron shells an atom has and the higher its atomic number, the less electronegative it is. According to this, Fluorine (F) at the top right corner of the periodic table is the most electronegative atom, and Francium (Fr) 🏥 at the bottom left corner is the least electronegative.

Resources
© 2024 Fiveable Inc. All rights reserved.
AP® and SAT® are trademarks registered by the College Board, which is not affiliated with, and does not endorse this website.