Transition state theory is crucial for understanding enzyme catalysis in biological systems. It explains how enzymes speed up reactions by stabilizing high-energy intermediates, lowering the activation energy barrier. This theory provides a quantitative framework for analyzing reaction rates and enzyme efficiency.
Enzymes use various strategies to stabilize transition states, including electrostatic interactions, hydrogen bonding, and conformational changes. By studying these mechanisms, scientists can design better enzyme inhibitors and drugs that mimic transition states, leading to more effective treatments for various diseases.
Transition State Theory Fundamentals
Key Concepts and Definitions
- Transition state theory is a theoretical framework used to describe the rates of chemical reactions, particularly for understanding the kinetics of enzymatic reactions in biological systems
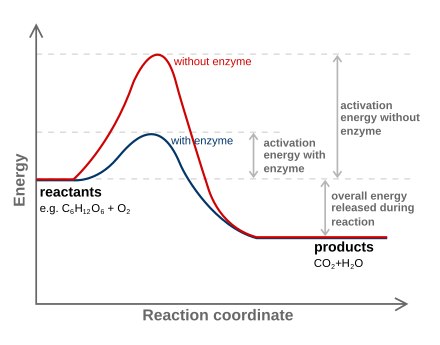
- The transition state is a high-energy, unstable intermediate structure formed during a chemical reaction, representing the highest energy point along the reaction coordinate
- The transition state is characterized by partial bond formation and breaking, with the reactants and products having equal potential to form from this state
- The rate of a chemical reaction is determined by the free energy difference between the reactants and the transition state, known as the activation energy ()
Quantitative Basis and Eyring Equation
- According to transition state theory, the rate of a reaction is proportional to the concentration of the transition state complex, which is in equilibrium with the reactants
- The Eyring equation relates the rate constant of a reaction to the Gibbs free energy of activation, temperature, and other thermodynamic parameters, providing a quantitative basis for transition state theory
- The Eyring equation is expressed as: , where is the rate constant, is the Boltzmann constant, is Planck's constant, is the absolute temperature, and is the gas constant
- The Eyring equation allows for the calculation of the activation energy and other thermodynamic parameters from experimental kinetic data (rate constants, temperature dependence)
- Transition state theory provides a framework for understanding the factors that influence reaction rates, such as temperature, pressure, and solvent effects
Enzyme Catalysis and Transition State Stabilization

Enzyme-Substrate Interactions and Transition State Formation
- Enzymes are biological catalysts that accelerate chemical reactions by lowering the activation energy barrier, thereby stabilizing the transition state
- Enzymes bind to substrates and form an enzyme-substrate complex, which undergoes conformational changes to form the transition state complex
- The active site of an enzyme is complementary to the structure of the transition state, providing a favorable environment for its formation and stabilization
- The binding energy released upon the formation of the enzyme-transition state complex is used to lower the energy barrier and increase the reaction rate
Strategies for Transition State Stabilization
- Enzymes employ various strategies to stabilize the transition state, such as electrostatic interactions, hydrogen bonding, and van der Waals forces, which contribute to the lowering of the activation energy
- Electrostatic interactions: Charged amino acid residues in the active site can stabilize the charge distribution of the transition state (aspartate, glutamate)
- Hydrogen bonding: Specific hydrogen bonds between the enzyme and the transition state can help orient and stabilize the intermediate (serine proteases)
- Van der Waals forces: Close packing of the active site around the transition state can maximize favorable van der Waals interactions and exclude water (hydrophobic pockets)
- The efficiency of enzyme catalysis is determined by the degree of transition state stabilization, which is influenced by the specific interactions between the enzyme and the transition state
- Enzymes may also employ strain and distortion to destabilize the ground state of the substrate, making it more energetically favorable to reach the transition state (lysozyme)
Enzyme Structure and Transition State Stabilization
Active Site Architecture and Transition State Complementarity
- The three-dimensional structure of an enzyme, particularly the active site, plays a crucial role in stabilizing the transition state and facilitating catalysis
- Enzymes possess specific amino acid residues in their active sites that interact with the transition state, providing a complementary environment for its stabilization
- The arrangement of amino acid residues in the active site creates a unique electrostatic and steric environment that favors the formation and stabilization of the transition state
- Electrostatic complementarity: The active site residues provide a charge distribution that complements the transition state (serine proteases, ribonuclease)
- Shape complementarity: The active site geometry closely matches the shape of the transition state, maximizing favorable interactions (carbonic anhydrase)
Conformational Changes and Transition State Stabilization
- Enzymes may undergo conformational changes upon substrate binding, which can further optimize the active site geometry for transition state stabilization
- Conformational changes can bring catalytic residues into proper alignment, exclude water from the active site, or create a favorable electrostatic environment (kinases, polymerases)
- Mutations in the active site residues can alter the enzyme's ability to stabilize the transition state, leading to changes in catalytic efficiency and specificity
- Comparative analysis of enzyme structures and their transition state analogues provides insights into the structural basis of transition state stabilization and aids in the design of enzyme inhibitors
Transition State Analogues in Enzyme Inhibition vs Drug Design
Transition State Analogues as Enzyme Inhibitors
- Transition state analogues are compounds that closely resemble the structure and electronic properties of the transition state of an enzymatic reaction
- These analogues bind tightly to the active site of an enzyme, mimicking the transition state and inhibiting the enzyme's catalytic activity
- Transition state analogues are valuable tools for studying enzyme mechanisms and identifying key interactions responsible for transition state stabilization
- The high affinity of transition state analogues for enzymes makes them potent inhibitors, with potential applications in drug design and therapeutics
- Transition state analogues can achieve binding affinities several orders of magnitude greater than substrate analogues (purine nucleoside phosphorylase inhibitors)
- Transition state analogues are often slow-binding or irreversible inhibitors due to their tight binding and slow dissociation rates (protease inhibitors)
Rational Design of Transition State Analogue Inhibitors
- Drugs designed as transition state analogues can effectively inhibit enzyme activity and modulate biological processes, such as in the treatment of viral infections, cancer, and metabolic disorders
- The rational design of transition state analogues involves detailed knowledge of the enzyme structure, reaction mechanism, and the electronic and geometric properties of the transition state
- Structure-based drug design approaches, such as X-ray crystallography and computational modeling, are employed to optimize the binding and specificity of transition state analogues
- X-ray crystallography provides high-resolution structures of enzyme-inhibitor complexes, revealing key interactions and guiding inhibitor optimization (HIV protease inhibitors)
- Computational methods, such as quantum mechanics and molecular dynamics simulations, can model the transition state and predict the binding of potential inhibitors (neuraminidase inhibitors)
- The development of transition state analogue inhibitors requires a multidisciplinary approach, integrating insights from enzymology, organic chemistry, structural biology, and medicinal chemistry
- Successful examples of transition state analogue inhibitors include HIV protease inhibitors (saquinavir), influenza neuraminidase inhibitors (oseltamivir), and purine nucleoside phosphorylase inhibitors (immucillin-H)